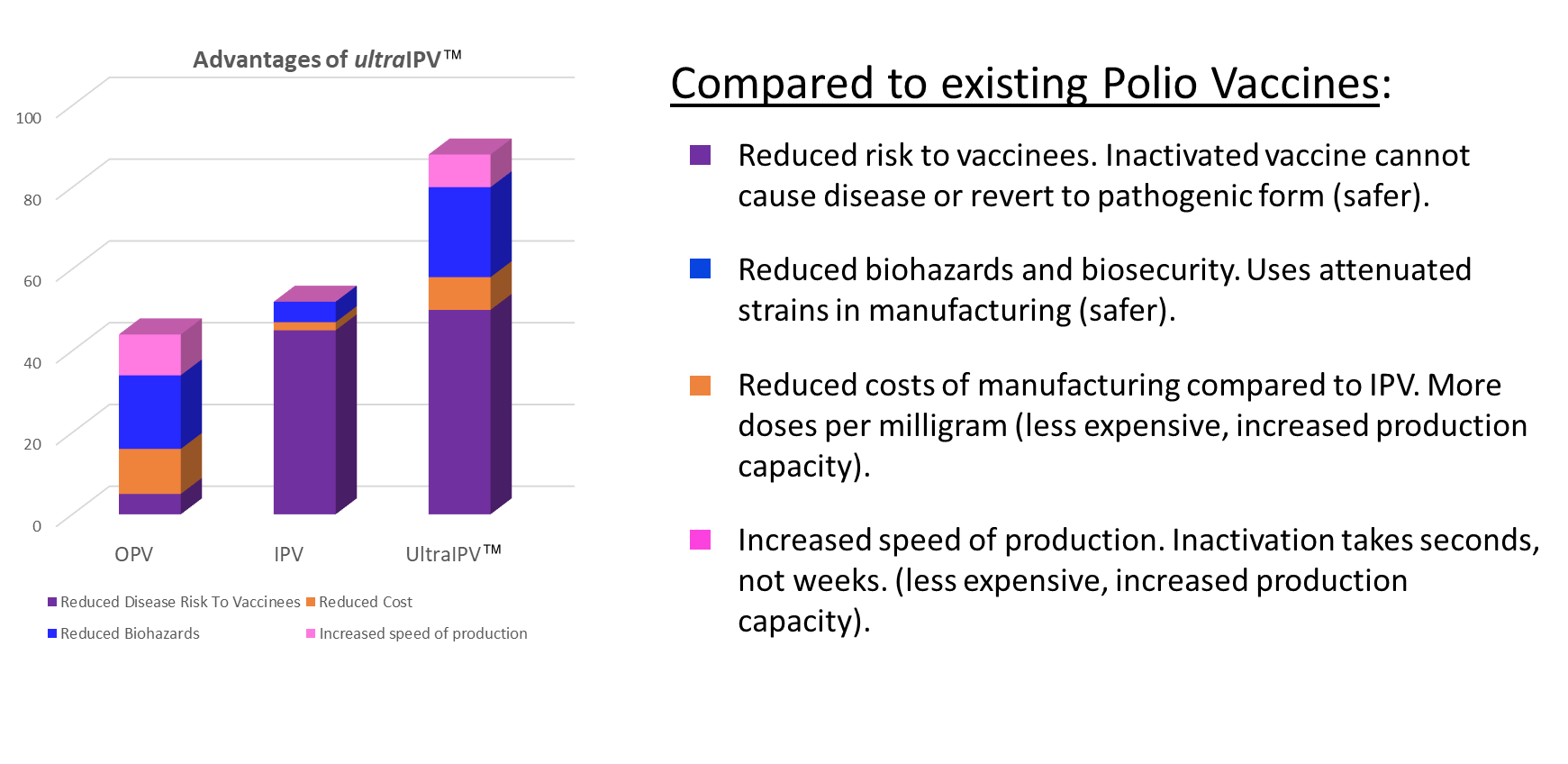
![]() The method allows the production of more doses per unit of purified viruses. The use of attenuated strains reduces biohazard and biosafety concerns surrounding the current production of inactivated polio vaccine (IPV) from pathogenic strains.
The method allows the production of more doses per unit of purified viruses. The use of attenuated strains reduces biohazard and biosafety concerns surrounding the current production of inactivated polio vaccine (IPV) from pathogenic strains.
Polio Types 2 and 3 have already been eradicated and efforts continue to eradicate Polio 1. Once eradicated, the WHO advises that countries continue to maintain vaccine vigilance for 20-30 years. After eradication, the biosafety concerns associated with manufacturing IPV from pathogenic strains will become increasingly serious.
 ultraIPV™ Provides:
ultraIPV™ Provides:
 Improved immunogenicity
Improved immunogenicity Greater Efficacy
Greater Efficacy Cost & time benefits and comparison against current/competing processes
Cost & time benefits and comparison against current/competing processes Reduced manufacturing costs
Reduced manufacturing costs Reduced Biosecurity threat
Reduced Biosecurity threat Safer for recipients and populations
Safer for recipients and populations

Let's Connect