![]() BMI has developed proprietary technology that overcomes immunological, technical, and safety issues associated with the current vaccines, while also enabling a more rapid and lower cost manufacturing process.
BMI has developed proprietary technology that overcomes immunological, technical, and safety issues associated with the current vaccines, while also enabling a more rapid and lower cost manufacturing process.
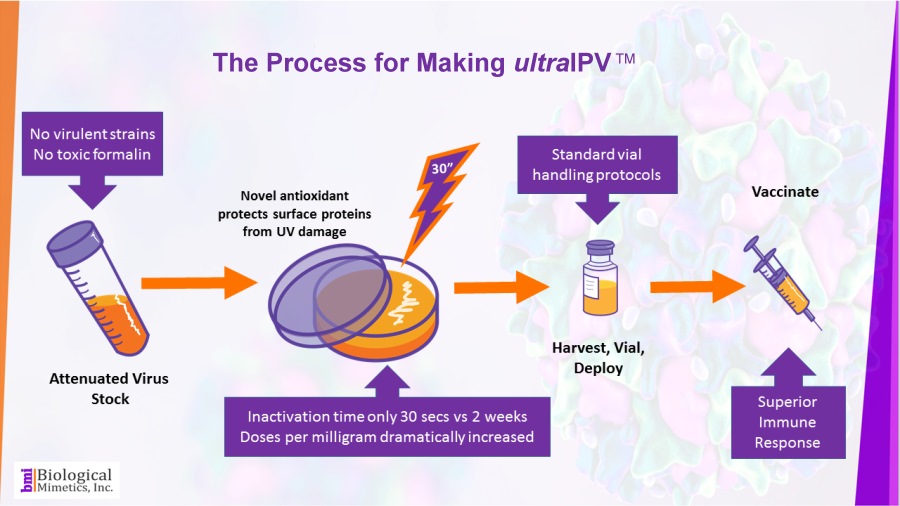
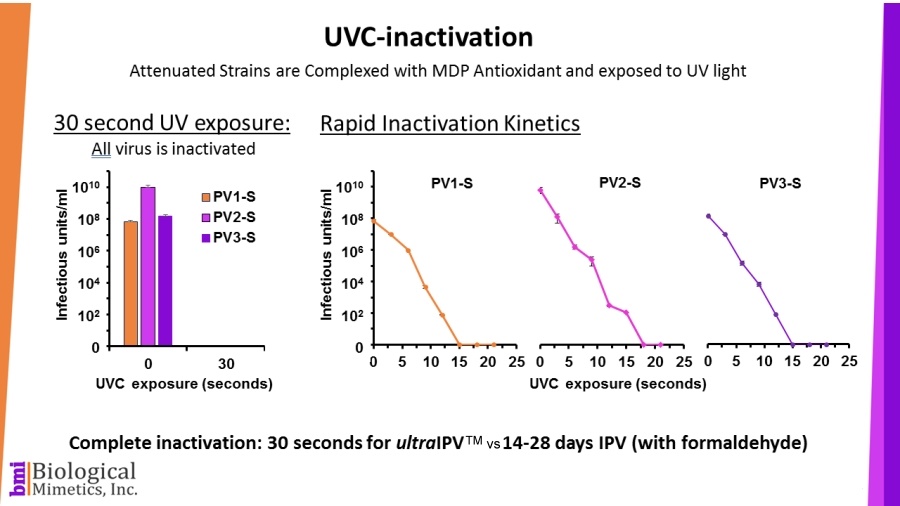
ultraIPV™ is produced using proprietary inactivation technology. Viruses are complexed with a manganese-decapeptide-phosphate (MDP) mixture and then exposed to UV light. A very quick, easy and reproducible process. The MDP system has been adapted from radiation-resistant bacteria such as Deinococcus radiodurans.
The use of attenuated Sabin strains to produce the vaccine greatly reduces safety and biosecurity concerns. This non-chemical inactivation process uses ultraviolet light resulting in reduced damage to the immunogenic surface features of the virus.
 BMI's process is rapid (less than a minute compared with 2-4 weeks for formalin-inactivation) and requires less downstream processing than IPV.
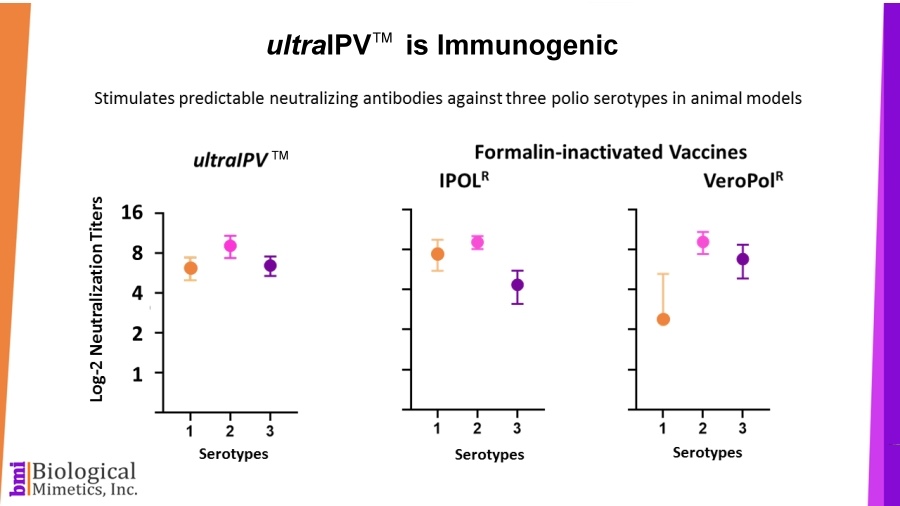
BMI's process is rapid (less than a minute compared with 2-4 weeks for formalin-inactivation) and requires less downstream processing than IPV. ultraIPV™ vaccine stimulates high titers of virus-neutralizing antibodies in animal models.
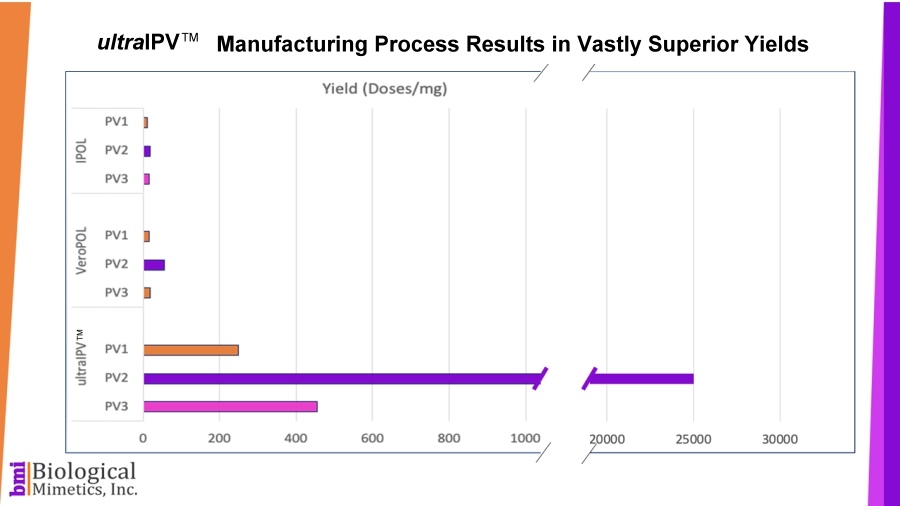
ultraIPV™ vaccine stimulates high titers of virus-neutralizing antibodies in animal models. Mass spectrometric analysis of ultraIPV™ and two commercially available IPV products indicate that many more doses of ultraIPV™ can be made than IPV per starting quantity of virus.
Mass spectrometric analysis of ultraIPV™ and two commercially available IPV products indicate that many more doses of ultraIPV™ can be made than IPV per starting quantity of virus. ultraIPV™ is expected to have a similar safety profile as IPV in vaccines with the exception that the use of formaldehyde is avoided.
ultraIPV™ is expected to have a similar safety profile as IPV in vaccines with the exception that the use of formaldehyde is avoided.
![]() The animations below illustrate:
The animations below illustrate:
 Chain 1 - VP1 - green; Chain 2 - VP2 - blue
Chain 1 - VP1 - green; Chain 2 - VP2 - blue Chain 3 - VP3 - red/pink; Chain 5 - VP3 - orange
Chain 3 - VP3 - red/pink; Chain 5 - VP3 - orange Chain 6 - VP3 - white; Chain 7 - VP2 - white
Chain 6 - VP3 - white; Chain 7 - VP2 - white  Chain 8 - VP1 - orange
Chain 8 - VP1 - orange

Let's Connect